Dr. Robert Green’s Vision for Genomic Medicine in Newborns: Turning Data into Diagnosis:
Dr. Robert Green, professor of genetics at Harvard Medical School, gave a landmark talk on making massive genomic datasets actionable—starting with newborns. While the idea of sequencing all newborns has existed for decades—since before the Human Genome Project began—it remains largely unimplemented in routine healthcare. Most U.S. hospitals still rely on “heel stick” testing, which covers only 30 to 75 conditions depending on the state. Meanwhile, hundreds of treatable genetic conditions remain undetected at birth.
BabySeq: The First Real-World Application
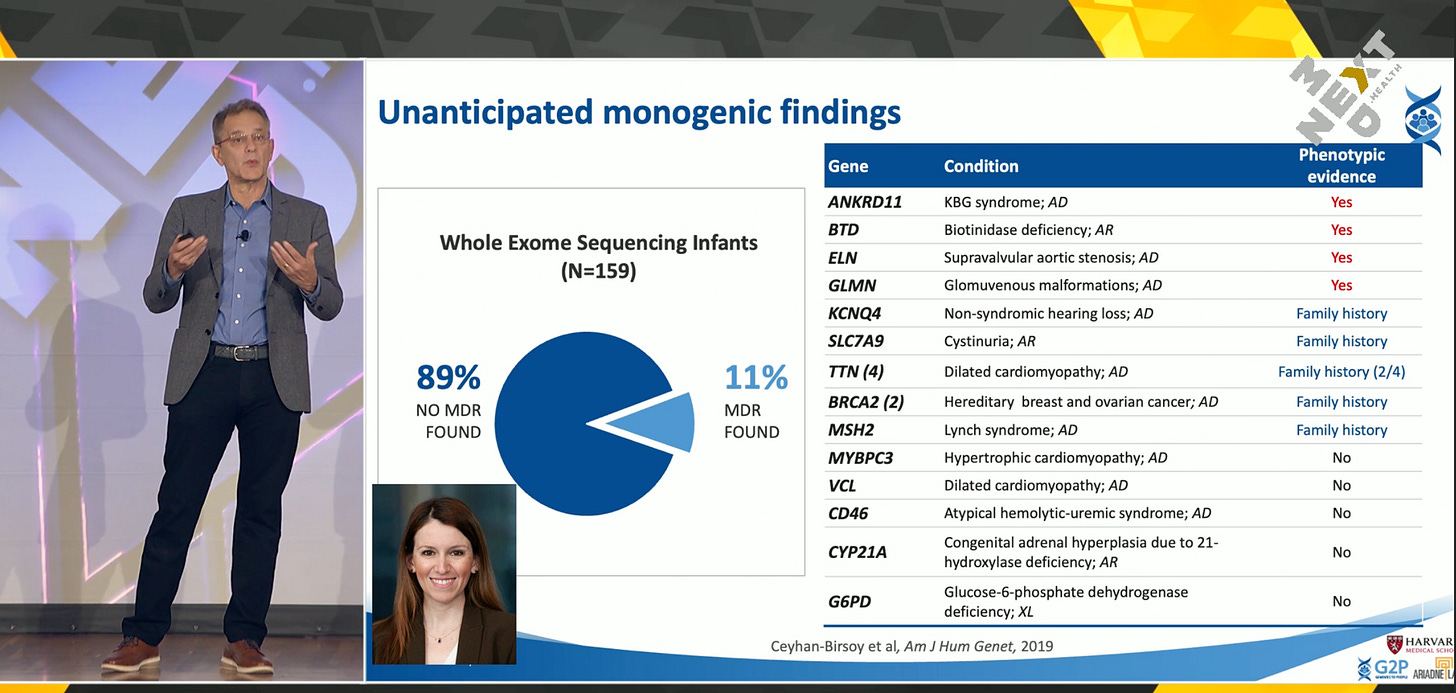
In 2015, Dr. Green and his team launched BabySeq, the first large-scale newborn sequencing study. The first participant was a four-day-old girl in Boston. Since then, the study has expanded to 12 sites across the country. What they discovered stunned even seasoned researchers: 11%—and later 12%—of newborns sequenced were found to carry monogenic mutations that put them at risk for serious diseases. In some cases, these children were already experiencing symptoms that had gone undetected by traditional screening or physical exams. Genomic data, therefore, was not just predictive—it was diagnostic.
Genomic Risk Across the Population
Dr. Green emphasized that nearly everyone carries some form of genetic risk. Analyzing roughly 5,000 disease-associated genes (the so-called medical exome) reveals that 15–20% of people carry dominant or bi-allelic recessive mutations linked to single-gene disorders. Most people are also carriers for recessive diseases, have atypical drug response profiles (pharmacogenomics), or carry elevated polygenic risk scores. While not everyone will develop a genetic disease, many unknowingly live with increased risk. Universal screening, especially at birth, could uncover and manage these risks much earlier.
Three Frontiers of Actionable Genomics
Dr. Green’s work spans three core areas in which genomic data becomes actionable:
Secondary Findings – For example, sequencing a child for heart issues and discovering a cancer risk mutation.
Return of Research Results – Providing study participants, such as those in the Framingham Heart Study, with medically relevant findings from their genomic data.
Population Screening – Sequencing healthy individuals proactively to identify health risks from birth or early life.
His lab, Genomes2People, leads globally in studying how to deliver these insights in ways that are scientifically rigorous, ethically responsible, and economically viable.
Addressing the Fear of Psychological Harm
One of the most common objections to newborn sequencing has been concern over psychological harm—both for parents and children. Critics feared that discovering serious genetic risks would create panic or permanently damage the parent-child relationship. To test this, Dr. Green’s team designed BabySeq as a randomized clinical trial and tracked psychological outcomes using validated metrics. The results showed no significant increases in anxiety, distress, or relational strain. Parents who opted in felt more empowered than burdened, seeing the information as a helpful tool rather than a cause for alarm.
Actionability: Treatment and Surveillance
A major highlight of the talk was Dr. Green’s two-tiered framework for actionability:
Treatability: The condition can be directly addressed through intervention, such as gene or cell therapies.
Surveillance: The condition may not be curable yet, but it can be monitored through ongoing screenings and follow-ups.
In BabySeq, every child identified with a concerning mutation fell into one or both of these categories. Some already showed symptoms of their condition. Others were monitored to catch early signs of disease progression. In either case, the data wasn’t theoretical—it led to tangible medical decisions.
The Ripple Effect: Diagnosing Parents Through Children
Unexpectedly, sequencing newborns often revealed health insights about their parents. In many cases, the same genetic mutation found in a child was traced to a parent who had been living with undiagnosed or misdiagnosed symptoms. For example, several parents carried TTN mutations linked to cardiomyopathy. Though they had heart disease, none had been properly diagnosed as having a genetic condition. In other cases, BabySeq helped uncover adult-onset cancer risks in mothers—such as BRCA mutations—based on their child’s DNA. Three mothers went on to receive life-saving preventive surgeries. What began as pediatric research ended up protecting entire families.
Cost Considerations and Health Economics
A concern among policymakers and clinicians is the cost of genomic screening. Would this type of precision medicine create expensive burdens, especially in a baby’s first year? Dr. Green’s team found that while sequencing did lead to a few extra tests or specialist visits, the overall cost impact was minor. In fact, when placed in the broader context of pediatric care, the incremental costs were negligible. More importantly, early identification often prevented much larger costs down the road—economically justifying the investment in many cases.
Systemic Barriers to Adoption
Despite its promise, widespread adoption of newborn sequencing remains stalled by structural challenges. Since 2008, only nine conditions have been added to the U.S. newborn screening panel. The state-by-state system is fragmented, underfunded, and slow to adapt. Dr. Green argued that public health infrastructure in this area is outdated and demoralized. If the U.S. is to lead the next wave of precision medicine, newborn sequencing must become standard—not a luxury. This would require national coordination, public education, and revised medical training. It’s a policy issue, not a technology problem.
Voices from the Families
Perhaps the most compelling part of the presentation came not from data, but from the voices of participating parents. “Why isn’t this done for everybody?” asked one mother. Another parent shared, “We just want our children to be healthy and happy. And if we can know something that could potentially harm them, we want to act on it.” These sentiments capture the human stakes of genomic screening. Far from being afraid, most families embraced the opportunity to be informed and proactive.
The Future: From Dream to Standard Practice
Dr. Green’s work stands at the intersection of possibility and pragmatism. He has taken a decades-old dream—universal genome sequencing—and brought it to life through careful, compassionate, data-driven research. He and his colleagues have demonstrated that sequencing newborns can do more than forecast health—it can catch undiagnosed diseases, prevent adult tragedies, and empower families to act. As genomic technologies become more accessible and gene therapies continue to advance, Dr. Green’s vision could become the standard for pediatric care in the 21st century.
The key barrier is not cost or feasibility—it’s culture and policy. Do we, as a society, have the will to embed genomic screening into the fabric of healthcare from day one? If the outcomes of BabySeq are any indication, the answer should be a resounding yes.